1999
2001
2002
2004
2005
2008
2010
2012
2015
2017
2018
2019
1999
NPI project started in 1999 with inspiration from the vision set by His Majesty Late Sultan Qaboos Bin Said to make Oman self reliant in medicine and to serve the people of Oman and the Gulf region, National Pharmaceutical Industries was established.

2001
2001 the project got approved by Ministry of Health Oman.

The factory was inaugurated by His Majesty Haitham bin Tariq Al Said

2002
NPI received approvals from all the GCC – KSA, UAE, Bahrain, Qatar, Kuwait and GHC.

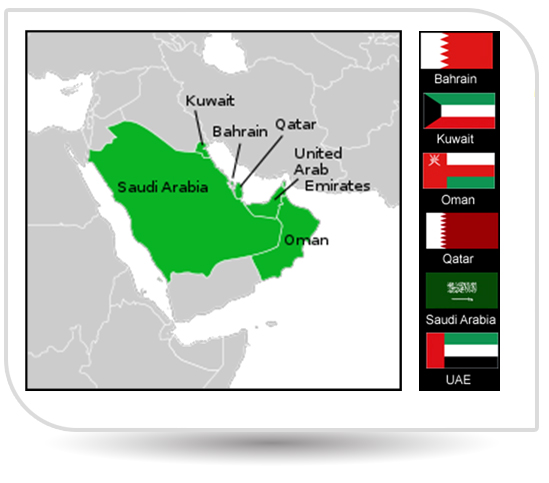
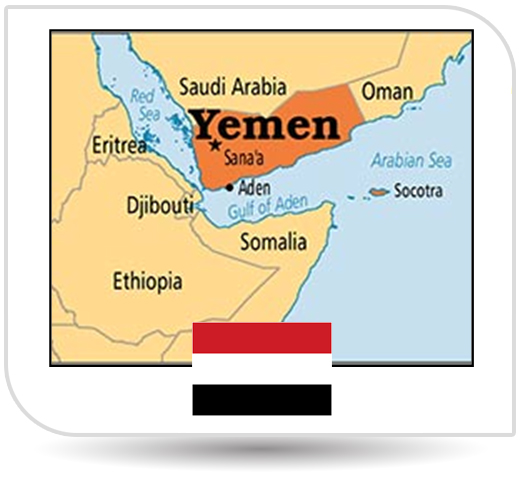
NPI also bagged the approval from Yemen

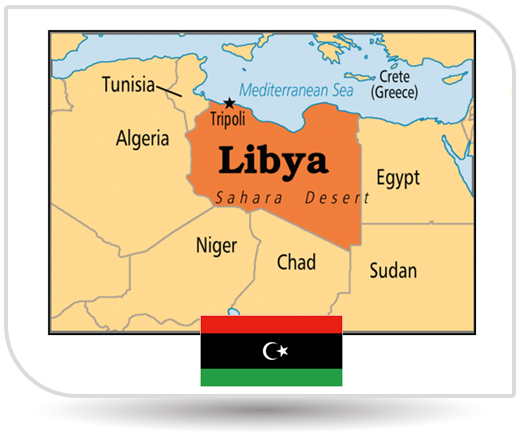
NPI also bagged the approval from Libya

NPI was the winner of Oman Award for Excellence “Investment Project of the Year 2002”

2004
NPI begin to expand its horizon in Africa by getting Approval for Burkina Faso

2005
NPI strengthened its operations in Middle East by getting the approval from the Jordan Ministry of Health.

2008
NPI with the vision of turning from local to global company registered in the Ivory Coast in Africa.

2010
NPI started expanding its operation in Africa by getting the approval of Kenya Health authority and truly moved in the direction of transforming to a global company.

NPI set a new bench mark in Quality by bagging ‘His Majesty Cup” award for Quality. NPI also received the Oman Green Habitat Award highlighting the blending of NPI in science and nature.

2012
NPI set up its front-end operations in Kingdom of Saudi Arabia – the biggest pharma market in Middle East

2015
NPI set up its front-end operations in United Arab Emirates– a highly dynamic market in in Middle East

2017
NPI further expanded its wings in Africa region by registering in Mauritius.

2018
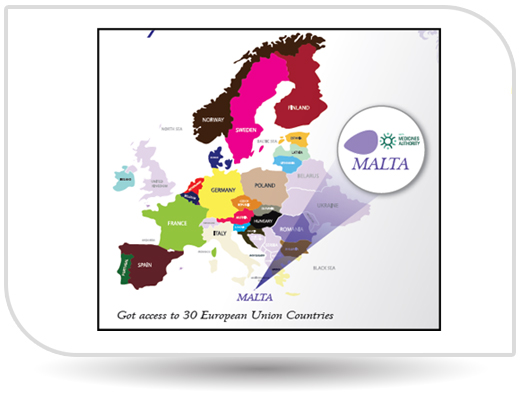
2018 was a remarkable year for NPI as it received the prestigious EUGMP certificate and got registered in the 30 European Union countries.

To add feather in the cap, NPI also received approval from Malawi

To add feather in the cap, NPI also received approval from Azerbaijan

2019
- NPI proved its stringent quality parameters by receiving the ISO 9001: 2015 accreditation.
- NPI received approval from Tanzania health authorities enabling it to strengthen its presence in Africa.
- NPI received approval from Uganda health authorities enabling it to strengthen its presence in Africa.
- NPI receives Ethiopia approval
- NPI receives GMP certificate from Ethiopia – Food and Health Care administration and control Authority
- NPI expanded it horizon by commencement of its state of art manufacturing Elixir Pharma in King Abdullah Economic City at Jeddah, KSA. The groundbreaking ceremony took place in Dec 2019 and was attended by its Board of Directors along with Oman and Saudi Health Ministry dignitaries.
- NPI also initiated the construction of its secondary packaging unit Elixir UAE at Dubai Science Park.
NPI proved its stringent quality parameters by receiving the ISO 9001: 2015 accreditation.

NPI received approval from Tanzania health authorities enabling it to strengthen its presence in Africa.

NPI received approval from Uganda health authorities enabling it to strengthen its presence in Africa.

NPI receives Ethiopia approval
NPI receives GMP certificate from Ethiopia – Food and Health Care administration and control Authority

NPI expanded it horizon by commencement of its state of art manufacturing Elixir Pharma in King Abdullah Economic City at Jeddah, KSA.
The groundbreaking ceremony took place in Dec 2019 and was attended by its Board of Directors along with Oman and Saudi Health Ministry dignitaries.